Description
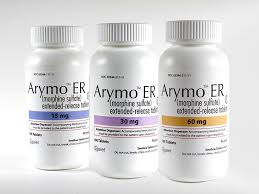
Buy ARYMO® ER (Morphine Sulfate) Cheap Price online
ARYMO® ER (Morphine Sulfate)Limitations of Use
- Because of the risks of addiction, abuse, and misuse with opioids, even at recommended doses, and because of the greater risks of overdose and death with extended-release opioid formulations, reserve ARYMO ER for use in patients for whom alternative treatment options (e.g., non-opioid analgesics or immediate-release opioids) are ineffective, not tolerated, or would be otherwise inadequate to provide sufficient management of pain.
- ARYMO ER is not indicated as an as-needed (prn) analgesic.
Advise both patients and caregivers about the risks of respiratory depression and sedation when ARYMO ER is used with benzodiazepines or other CNS depressants (including alcohol and illicit drugs). Advise patients not to drive or operate heavy machinery until the effects of concomitant use of the benzodiazepine or other CNS depressant have been determined. Screen patients for risk of substance use disorders, including opioid abuse and misuse, and warn them of the risk of overdose and death associated with the use of additional CNS depressants, including alcohol and illicit drugs.
CONTRAINDICATIONS
ARYMO ER is contraindicated in patients with:
- Significant respiratory depression
- Acute or severe bronchial asthma in an unmonitored setting or in the absence of resuscitative equipment
- Concurrent use of monoamine oxidase inhibitors (MAOIs) or use of MAOIs within the last 14 days
- Known or suspected gastrointestinal obstruction, including paralytic ileus
- Hypersensitivity (eg, anaphylaxis) to morphine or naltrexone
WARNINGS AND PRECAUTIONS
Opioid Analgesic Risk Evaluation and Mitigation Strategy (REMS)
To ensure that the benefits of opioid analgesics outweigh the risks of addiction, abuse, and misuse, the Food and Drug Administration (FDA) has required a Risk Evaluation and Mitigation Strategy (REMS) for these products. Under the requirements of the REMS, drug companies with approved opioid analgesic products must make REMS-compliant education programs available to healthcare providers. Healthcare providers are strongly encouraged to do all of the following:
- Complete a REMS-compliant education program offered by an accredited provider of continuing education (CE) or another education program that includes all the elements of the FDA Education Blueprint for Health Care Providers Involved in the Management or Support of Patients with Pain.
- Discuss the safe use, serious risks, and proper storage and disposal of opioid analgesics with patients and/or their caregivers every time these medicines are prescribed. The Patient Counseling Guide (PCG) can be obtained at this link: www.fda.gov/OpioidAnalgesicREMSPCG.
- Emphasize to patients and their caregivers the importance of reading the Medication Guide that they will receive from their pharmacist every time an opioid analgesic is dispensed to them.
- Consider using other tools to improve patient, household, and community safety, such as patient-prescriber agreements that reinforce patient-prescriber responsibilities.
To obtain further information on the opioid analgesic REMS and for a list of accredited REMS CME/CE, call 1-800-503-0784, or log on to www.opioidanalgesicrems.com. The FDA Blueprint can be found at www.fda.gov/OpioidAnalgesicREMSBlueprint.
Life-threatening Respiratory Depression in Patients With Chronic Pulmonary Disease or in Elderly, Cachectic, or Debilitated Patients
The use of ARYMO ER in patients with acute or severe bronchial asthma in an unmonitored setting or in the absence of resuscitative equipment is contraindicated.
Patients With Chronic Pulmonary Disease: ARYMO ER-treated patients with significant chronic obstructive pulmonary disease or cor pulmonale, and those with a substantially decreased respiratory reserve, hypoxia, hypercapnia, or pre-existing respiratory depression are at increased risk of decreased respiratory drive, including apnea, even at recommended dosages of ARYMO ER.